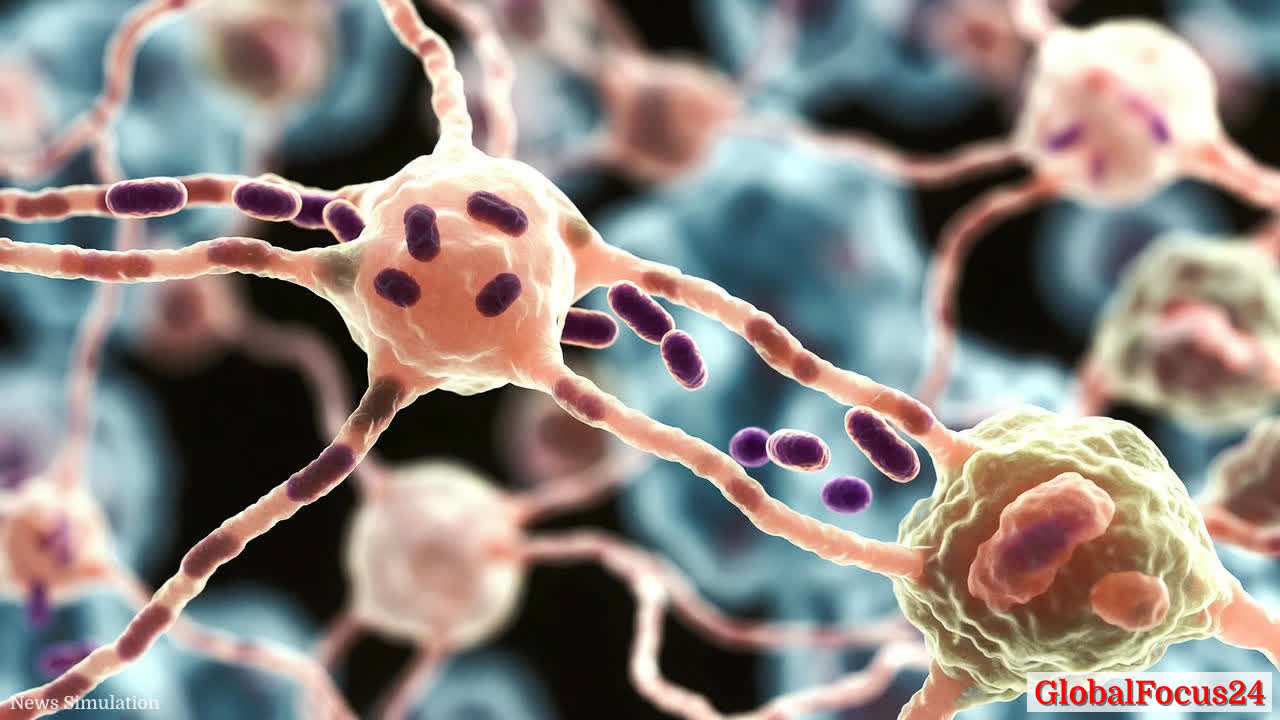
Neurons Transfer Mitochondria to Tumor Cells, Boosting Cancer Spread
A collaborative study across neuroscience and oncology fronts has revealed a surprising mechanism by which tumors accelerate their spread: neurons can donate mitochondria directly to cancer cells. This mitochondrial handoff appears to empower cancer cells with enhanced energy production and resilience, enabling more aggressive invasion and metastatic potential. The finding adds a crucial piece to the evolving puzzle of cancer neuroscience, a field that explores how nerve cells and signaling systems within the tumor microenvironment influence cancer initiation, growth, and dissemination.
Historical context and the emergence of cancer neuroscience The concept that the nervous system can influence cancer dates back several decades, with early observations linking nerve growth to tumor aggressiveness in certain cancers. For years, researchers noted that tumors often co-opt neural pathways to support growth and migration, a relationship that became more scientifically precise through advances in molecular biology and imaging. The modern era of cancer neuroscience emphasizes bidirectional communication: cancer cells send signals that remodel neural circuits, while neurons and glial cells release factors that can nourish tumor cells, create protective niches, or guide metastatic cells to distant sites. The latest work on mitochondrial transfer fits squarely within this broader narrative, offering a concrete cellular mechanism by which nerves may directly bolster malignant behavior.
Mechanism of mitochondrial transfer and its implications The study identifies a process in which tumor-associated neurons physically transfer mitochondria to adjacent cancer cells. Mitochondria, the energy-producing organelles of the cell, are central to cellular metabolism and survival under stress. When neurons donate mitochondria to cancer cells, the recipient cells gain access to enhanced oxidative phosphorylation capacity and improved energy resilience. This can translate into several downstream effects:
- Increased proliferation and survival under hostile conditions, including nutrient fluctuations and therapeutic pressure.
- Enhanced migratory and invasive capabilities, enabling cancer cells to traverse tissue barriers and colonize new tissues.
- Greater resistance to certain treatments, particularly those that disrupt cellular energy production or induce metabolic stress.
The exact signaling cues that trigger mitochondrial donation remain an area of active investigation. Researchers are examining how neuronal health, synaptic activity, and the tumor’s own metabolic state influence the frequency and efficiency of mitochondrial transfer. Early data suggest that neuronal activity and specific surface proteins may mediate the physical transfer event, while cancer cells may selectively uptake healthy mitochondria to sustain high-energy demands during invasion.
Regional patterns and cross-cancer relevance While the mechanism was identified in controlled experimental models, its implications span multiple cancer types and anatomical contexts. Tumors that commonly interact with nerve fibers—such as those in the pancreas, prostate, head and neck, and certain brain cancers—are likely to be most affected by this mitochondrial exchange. In these regions, dense neural networks interface with tumor masses, creating opportunities for mitochondrial handoffs that bolster metastatic potential. The regional relevance of this mechanism is underscored by the fact that nerve-rich tumor microenvironments often correlate with more aggressive disease and poorer patient outcomes, reinforcing the need to consider neural-cancer interactions in diagnostic and therapeutic strategies.
Economic impact and potential clinical implications From an economic standpoint, advances in cancer neuroscience have the potential to influence healthcare costs, pharmaceutical development, and regional treatment paradigms. If mitochondrial transfer proves to be a generalizable driver of metastasis across cancer types, therapies that disrupt this exchange could become a new class of anti-metastatic agents. Such interventions might include:
- Targeting neuron-tumor interfaces to prevent mitochondrial donation, potentially reducing metastatic spread and improving surgical and radiologic outcomes.
- Developing mitochondrial delivery inhibitors that selectively impair the uptake or integration of exogenous mitochondria by cancer cells.
- Combining neural-immune-modulating approaches with metabolic therapies to address both tumor signaling networks and energy dependencies.
Healthcare systems could see shifts in resource allocation as these strategies are refined, with potential reductions in late-stage metastasis-related care and improved quality of life for patients facing aggressive cancers. Biotech and pharmaceutical sectors may prioritize research programs that map neuron-tumor interactions and identify druggable targets at the interface of neural signaling and cancer metabolism.
Comparative perspectives across regions Geographic variation in cancer incidence, neural innervation patterns, and access to advanced diagnostics shapes how new mechanisms translate into practice. Regions with high prevalence of nerves-tcarrying tumor interactions may benefit from earlier adoption of diagnostic tools that assess neural involvement in tumors. Conversely, areas with limited access to comprehensive cancer neuroscience research could experience slower incorporation of neural-targeted therapies into standard care. International collaboration and data-sharing initiatives will be essential to determine how widely applicable mitochondrial transfer is across populations and healthcare settings.
Public reaction and the path forward Public awareness of cancer biology often focuses on conventional treatment modalities, yet discoveries that illuminate the neural dimension of cancer can heighten interest in multidisciplinary approaches. Patients and caregivers may welcome research that opens new therapeutic options while also signaling the complexity of cancer as a systemic disease influenced by nervous system activity. As researchers refine the understanding of mitochondrial transfer, clinicians will need to translate these insights into safe, effective strategies that integrate with existing regimens such as surgery, radiation, chemotherapy, and emerging targeted therapies.
Research trajectories and methodological considerations The scientific community is pursuing several parallel directions to validate and extend these findings:
- In vivo studies to confirm that mitochondrial transfer from neurons to cancer cells occurs in human tumors and correlates with metastatic patterns.
- Molecular profiling to identify the proteins and signaling pathways that mediate mitochondrial donation and uptake.
- Evaluation of therapeutic windows to minimize potential adverse effects on normal neuronal function while impeding cancer cell energetics.
Ethical and safety considerations will guide the translation of neural-targeted therapies, ensuring that interventions do not compromise essential nervous system activities or neurological health. Regulatory frameworks will need to adapt as novel classes of therapies targeting neuron-tumor interfaces enter clinical trials.
Historical parallels and broader scientific context This discovery echoes a broader scientific pattern: cells often exchange cytoplasmic components in ways that rewire biology. Mitochondrial donation has been observed in other contexts, such as stem cell biology and intercellular transfer between diverse cell types. The novelty here lies in the intentionality and relevance of mitochondrial transfer within the tumor microenvironment, directly contributing to cancer cell energetics and metastatic capacity. If confirmed across multiple tumor types, this mechanism could reshape fundamental theories of tumor progression and highlight the nervous system as an active participant in cancer ecology rather than a passive backdrop.
Concluding reflections The revelation that neurons can donate mitochondria to cancer cells adds a compelling Chapter to the evolving story of cancer neuroscience. By enhancing the metabolic toolkit available to malignant cells, mitochondrial transfer from tumor-associated neurons offers a plausible driver of metastasis and therapeutic resistance. As researchers expand the evidence base, the medical community will be watching closely for translational opportunities that could disrupt this exchange, potentially slowing the spread of disease and improving outcomes for patients facing aggressive cancers. The interplay between neural signaling and cancer metabolism underscores the importance of interdisciplinary collaboration in tackling one of medicine’s oldest and most challenging frontiers.