Breakthrough Atlas Reveals Cellular Secrets of Human Reproductive Development
A new, large-scale cellular atlas maps the developing human reproductive tract from 6 to 21 weeks post-conception, offering an unprecedented view of how male and female reproductive systems form at the cellular level. By combining single-cell RNA sequencing, spatial transcriptomics, and chromatin accessibility data, researchers have identified 52 distinct cell types and traced their dynamic interactions across critical windows of fetal development. The atlas provides a comprehensive framework for understanding normal development, congenital anomalies, and infertility, while also highlighting opportunities for improved in vitro models and potential interventions in reproductive health.
Historical context: building a map of human reproductive development
For decades, scientists have sought a detailed, cell-by-cell understanding of how the reproductive tract differentiates in utero. Early anatomical studies laid the groundwork by describing the Müllerian ducts, which give rise to female internal reproductive organs, and the Wolffian ducts, which contribute to male reproductive structures. However, translating macroscopic anatomy into cellular and molecular mechanisms required new technologies. The advent of single-cell sequencing and advanced imaging opened the door to a high-resolution view of tissue formation, enabling scientists to observe how gene expression, cell fate decisions, and tissue patterning unfold in real time. This atlas marks a milestone by integrating multiple modalities to create a spatiotemporal blueprint of human reproduction during a critical developmental period.
Key findings: cell types, lineage trajectories, and regional patterning
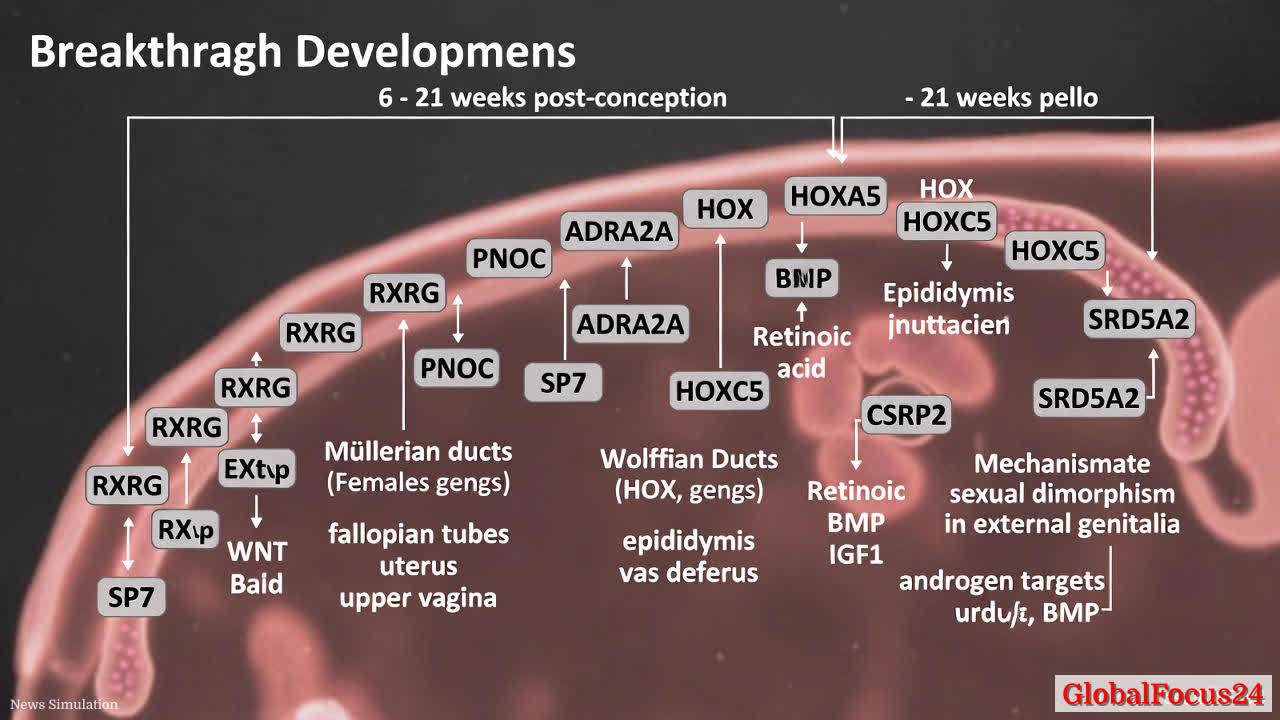
- 52 distinct cell types identified: The atlas catalogues a diverse array of cell populations across the developing reproductive tract, including epithelial, mesenchymal, endothelial, and immune-like cells. Each cell type carries a unique transcriptional signature and spatial location that informs its function in organ formation.
- Coelomic epithelium as a developmental origin: Trajectories tracing the coelomic epithelium reveal early-upregulated genes such as RXRG and PNOC during the onset of Müllerian duct development, highlighting initial molecular cues that steer the formation of female internal structures.
- Distinct male and female trajectories: The study maps male-specific markers in degenerating mesenchyme, including ADRA2A and SP7, which accompany the regression of certain structures in the male lineage. Conversely, female pathways emphasize Müllerian duct maintenance and differentiation into fallopian tubes, uterus, and upper vagina.
- Role of HOX genes in rostrocaudal patterning: Unexpectedly, thoracic HOX genes such as HOXA5 and HOXC5 are prominent in rostral regions of the fallopian tube and epididymal mesenchyme. This finding underscores a conserved role for HOX-driven gradients in establishing regional identities along the reproductive tract.
- Gradients of signaling pathways: The atlas reveals spatial gradients of key signaling molecules. WNT and retinoic acid signaling shape the tubes and uterus, while BMP and IGF1 signaling influence the vaginal region. These gradients help organize tissue identity and functional maturation along the rostrocaudal axis.
- Early epithelial regionalization and function: Epithelial cells in the fallopian tube and epididymis show regional specialization early in development, supporting functions such as sperm maturation and fertilization competence. Uterocervical canal regionalization occurs later, aligning with shifts in tissue protection, mucus production, and barrier function.
- External genitalia development and sexual dimorphism: The data illustrate that external genitalia do not harbor sex-specific cells per se. Instead, androgen-driven targets—such as CSRP2 and SRD5A2—drive male urethral development through Notch signaling and adherens junction pathways. This emphasizes a hormonally mediated mechanism for sexual differentiation of external structures rather than a broad transcriptional sex distinction at the cellular level.
- Endocrine disruptor vulnerability in fetal uterine epithelium: Organoid models derived from fetal uterine epithelium show heightened sensitivity to endocrine disruptors like bisphenol A (BPA) and butyl benzyl phthalate (BBP). These compounds increase ciliogenesis and upregulate estrogen-responsive genes, illustrating a mechanism by which environmental exposures could influence development.
Economic impact: implications for health, industry, and research
- Advancing infertility research: By pinpointing precise cellular windows of susceptibility and identifying early markers of normal development, the atlas informs strategies to model infertility in vitro. Better models could accelerate drug discovery, improve screening for teratogenicity, and enable targeted therapies to address developmental abnormalities.
- Congenital anomalies and clinical care: Improved understanding of how disorders arise at the cellular level can guide diagnostic approaches and prenatal care. Early detection of atypical development patterns may improve outcomes through timely interventions and monitoring.
- Biotech and pharmaceutical applications: The integrated dataset serves as a rich resource for companies developing organoids, regenerative therapies, or reproductive health products. Clear maps of cell types and signaling cues facilitate the design of more accurate tissue models and screening platforms.
- Public health and regulatory considerations: Evidence of environmental compounds affecting fetal uterine epithelium underscores the importance of safeguarding maternal and fetal health. Policymakers and regulators may leverage such findings to refine exposure guidelines and promote safer chemical use.
Regional comparisons: context across populations and anatomy
- Regional consistency in developmental programs: Across studied samples, core patterning signals, including HOX-driven regionalization and key signaling pathways, appear conserved, suggesting robust, evolutionarily conserved mechanisms guiding reproductive tract formation.
- Variation in timing and maturation: While fundamental pathways are shared, regional timing of epithelial differentiation and duct canalization exhibits subtle differences that may reflect genetic background, maternal health, and environmental exposures. Understanding these nuances can improve interpretation of prenatal imaging and diagnosis.
- Cross-species perspective: The atlas aligns with known mammalian developmental paradigms, reinforcing the relevance of animal models while highlighting unique human-specific regulatory features. This dual insight supports translational research while acknowledging species differences in pregnancy biology.
Structure and methods: how the atlas was built
- Multi-omics integration: Researchers combined single-cell RNA sequencing to map gene expression, spatial transcriptomics to locate cells within tissue architecture, and chromatin accessibility data to infer regulatory landscapes. This triad enables a holistic view of both cellular identity and regulatory control.
- Spatiotemporal resolution: Sampling from 6 to 21 weeks post-conception captures early organogenesis, canalization events, and maturation processes that set the stage for lifelong reproductive health. The high-resolution temporal dimension helps identify when certain lineages diverge and how key genes drive differentiation.
- Data interpretation and validation: The study uses computational trajectory analysis to infer lineage relationships and validates findings with independent markers and, where possible, functional assays in organoid or in vitro models. This combination strengthens confidence in the inferred regulatory networks.
Applications for clinical research and education
- Diagnostic biomarkers: The identification of lineage-specific markers and regulatory nodes provides candidate biomarkers that could help in diagnosing developmental disorders of the reproductive tract, both prenatally and postnatally.
- Organoid model development: The atlas informs the design of organoids that recapitulate early reproductive tract development. These models could be used to study gene function, test environmental toxins, or screen therapeutics in a human-relevant context.
- Training and public understanding: The atlas offers a rich educational resource for clinicians, researchers, and students. Clear maps of cell types and their spatial relationships help demystify complex developmental biology and support training in obstetrics and pediatric specialties.
Potential limitations and future directions
- Scope of developmental window: While 6 to 21 weeks covers critical formation stages, some later maturation events and postnatal adaptations remain to be explored. Extending the temporal range could reveal additional regulatory dynamics relevant to puberty and adult reproductive health.
- Functional validation: Observational maps are powerful, but functional experiments in human tissues are limited by ethical and technical constraints. Ongoing efforts to develop safe, ethically sourced models will be essential to validate causality for key regulatory interactions.
- Population diversity: Expanding studies to include diverse genetic backgrounds will help determine the universality of observed patterns and identify population-specific variations that may influence reproductive development and disease risk.
Public reaction and health communication
- Reassurance about fundamental biology: The atlas reinforces a sense of coherence in human development, showing that many developmental programs are tightly regulated and conserved. This is reassuring for the medical community and the public, as it supports predictable outcomes in normal development.
- Awareness of environmental risks: The demonstration of sensitivity to certain endocrine disruptors in fetal tissues may prompt heightened public health messaging and increased emphasis on reducing exposure to harmful compounds during pregnancy.
- Empowering patient advocacy: With clearer explanations of how congenital anomalies arise, advocacy groups can better communicate risks, advocate for research funding, and support families affected by reproductive development disorders.
Conclusion: a new era for reproductive biology and health
The breakthrough atlas represents a transformative step in reproductive biology, providing a detailed, cell-level map of human fetal development that bridges molecular mechanisms, tissue architecture, and functional outcomes. By integrating multiple data modalities, the atlas illuminates how regional identity and sex-specific differentiation emerge from shared progenitors, guided by a choreography of signaling pathways and transcriptional regulators. The implications extend beyond basic science to clinical research, diagnostic innovation, and public health, offering a foundation for improved models, targeted interventions, and informed policy decisions that protect reproductive health from the earliest stages of life.